CAR T-cell therapy
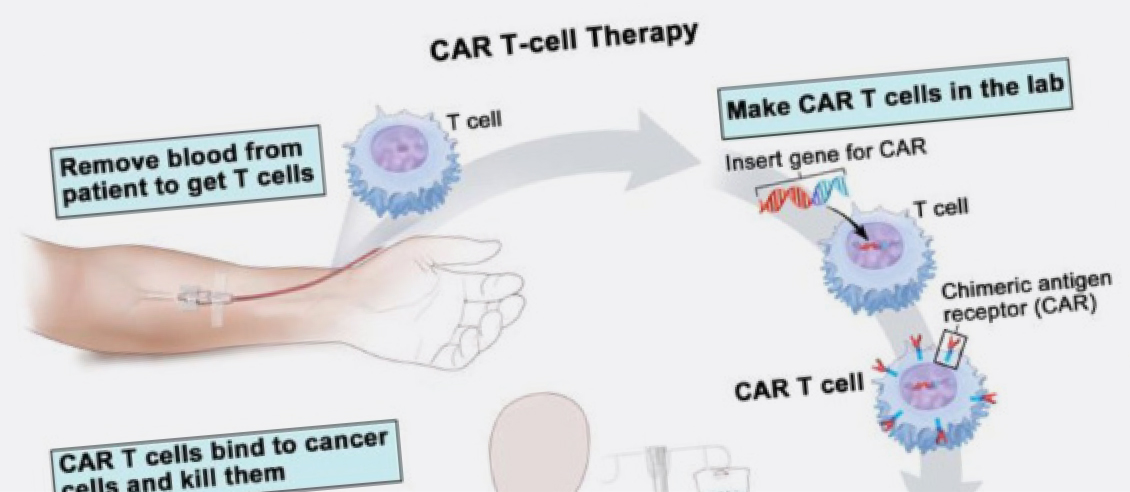
In December 2018 the Australian Therapeutic Goods Administrator (TGA) approved the use of chimeric antigen receptor T cell (CAR-T cell) therapy for two types of blood cancer, diffuse large B cell lymphoma (DLBCL) and B cell precursor acute lymphoblastic leukaemia (ALL). CAR-T cell therapy involves extracting some of the patients T-cells (white blood cells that form part of our immune system) and genetically altering them in the laboratory. The alteration to the cells involves adding a receptor to the T-cells that attach to a protein found on the patient’s cancer cell. Once the T-cell is attached to the cancer cell it initiates cell death.
To hear about CAR-T cell from an expert and the promise it holds for Australian blood cancer patients, listen to Snowdome Fellowship recipient Dr Michael Dickinson.
