Understanding clinical trials
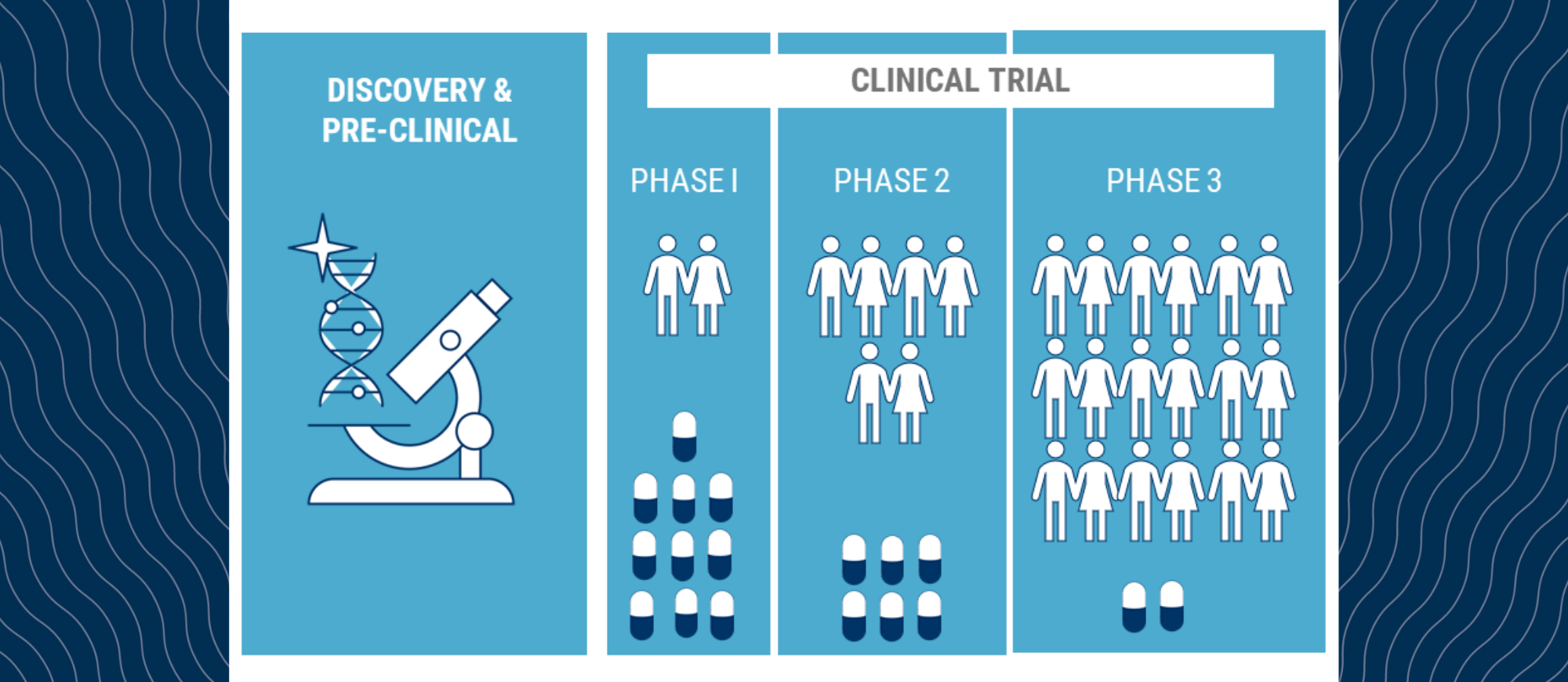
Clinical trials are often the gateway to new treatments for patients with blood cancer. When new therapies are being tested they go through various stages. If you have ever wondered about the various clinical trial stages and what they mean, below is a synopsis to provide you with some insight.
Phase I trials are the first human trials where patient numbers are small, and researchers are determining the best dose of a new treatment for the right balance between effectiveness and side effects. Usually, a wide range of doses are tested.
Phase II trials involve a larger number of patients. Researchers tend to study fewer doses based on the results of the Phase I studies. The trial is designed to provide researchers with an idea of the best dose to take to market.
Phase III trials include larger number of patients again and only one or two doses are tested. The trial usually compares the new treatment to standard of care to determine if the new treatment is at least as effective and safe as current treatments. The results of the Phase III trial are used to register the treatment with the respective health authority. In Australia, that group is known as the Therapeutic Goods Administration (TGA).
Clinical trials involve a huge amount of work and require a team of people at the hospital to manage the trial. There is patient selection and screening, data entry, clinical trial monitoring, assessing patients at check-up for the various trial end points and of course there is the patient’s time. It is important to know that if you are enrolled in a trial, you have the right to stop being part of the trial at any time. Now days, new cancer treatments being tested are generally well tolerated as we move towards more precision treatments. So, while you may feel like a human guinea pig, chances are you will be receiving a treatment that is well tolerated. Not all clinical trials test a brand new treatment. Sometimes the treatment is already approved for use but in a different type of cancer or for a different disease. This is becoming more common as we uncover various mutations that are common across different cancers.
As trials involve a large team and often frequent hospital visits, more clinical trials for blood cancer have been offered to hospitals in Australia and New Zealand this past year due to our handling on the COVID-19 pandemic. Hospitals in Europe and the U.S. have simply not been able to conduct trials. This is a wonderful opportunity for blood cancer patients and for our wonderful researchers and clinicians who can demonstrate to the world how good we are at conducting trials.
